INBRAIN Neuroelectronics announces FDA Breakthrough Device designation for its graphene-based intelligent network modulation platform
A first-of-a-kind device, the INBRAIN Intelligent Network Modulation System is designed to offer a new treatment option for patients with Parkinson’s disease. It harnesses the properties of graphene and the power of machine learning software to provide highly targeted and adaptive neuroelectronic therapy. INBRAIN Neuroelectronics is a health-tech company that originated as a spin-off of the Catalan Institute of Nanoscience and Nanotechnology (ICN2) and ICREA, with the participation of the IMB-CNM-CSIC.
Original news published at ICN2
INBRAIN Neuroelectronics S.L., a health-tech company dedicated to developing the world’s first smart graphene-neural platform, today announced that its Intelligent Network Modulation System has been granted the Breakthrough Device Designation (BDD) from the U.S. Food & Drug Administration (FDA) as an adjunctive therapy for treating Parkinson’s disease. INBRAIN Neuroelectronics is a spin-off company originated from the Catalan Institute of Nanoscience and Nanotechnology –specifically, the Advanced Electronic Materials and Devices Group led by Prof. José Antonio Garrido and the Nanomedicine Group led by Prof. Kostas Kostarelos– and ICREA, with the participation of the IMB-CNM-CSIC.
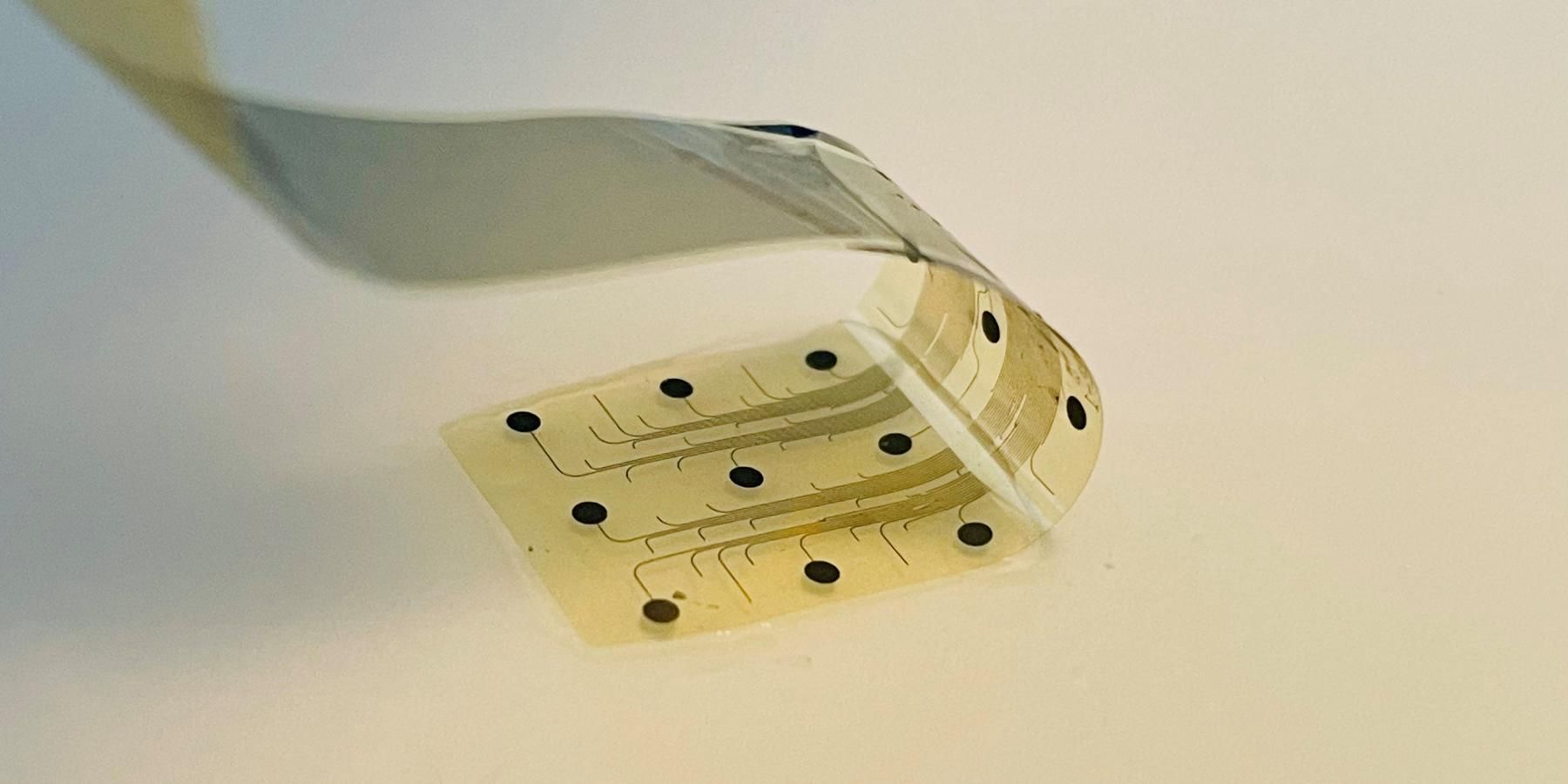
The INBRAIN system harnesses the power of graphene, a two-dimensional material made of a lattice of carbon atoms only one atom thick. The thinnest material known, yet stronger than steel, graphene exhibits a unique combination of electrical and mechanical properties that makes it ideal for neurotechnology innovation. INBRAIN’s neural platform technology enables ultra-high signal resolution at levels never achieved before and uses machine learning software that decodes therapy-specific biomarkers. This information is exploited to provide highly targeted and adaptive neuroelectronic treatments aimed at rebalancing pathological neural networks.
“This Breakthrough Device Designation from the FDA signifies the potential of the INBRAIN neural platform to further improve the lives of patients with Parkinson’s disease,” commented Dan Gnansia, INBRAIN Neuroelectronics Head of Clinical Affairs. “We look forward to working with the agency to help bring this important advance into clinical practice.”
The BDD recognizes devices that represent a major innovation or offer reasonable evidence of significant advantage over existing approved or cleared alternatives. It also expedites the development and FDA review of new devices with the potential to more effectively treat or diagnose life-threatening or irreversibly debilitating conditions.
“From the current available clinical brain interfaces, deep brain stimulation (DBS) is a successful therapy to treat Parkinson’s disease. However, current leads have restrictions regarding their relatively large size and low density, limiting their precision for targeting of small deep structures such as the subthalamic nucleus (STN), and the spatial or signal resolution when sensing the local brain electrical activity,” explained Helen Bronte Stewart, professor of neurology and neurological sciences at Stanford University School of Medicine. “INBRAIN’s new generation of ultrathin graphene-based high resolution interfaces and associated network platform may vastly improve the precision, efficiency and efficacy of DBS and closed-loop or adaptive modulation. The FDA Breakthrough Device designation is a statement to how this technology may be a paradigm shift in the scope of neuromodulation for people with Parkinson’s disease and hopefully for other neuropsychiatric conditions in the future.”
Thanks to the FDA Breakthrough Device recognition of the INBRAIN system, the company will receive feedback throughout the regulatory process, as well as prioritized review by the agency.
“INBRAIN is dedicated to leveraging new discoveries in materials science and transforming them into safe and effective breakthrough therapy applications,” added Carolina Aguilar, INBRAIN Neuroelectronics CEO and co-founder “We anticipate developing our technology to treat other conditions affecting the central and peripheral nervous systems to make BCI technology relevant in neuro and bioelectronics.”




